 Family studies and studies on twins have clearly shown that both psoriasis and psoriatic arthritis have a great genetic component. People with a fist degree relative suffering from psoriasis are much more likely to start suffering from the disease if compared to the general population. In particular, the risk of psoriasis in patients’ children is 4 to 10 times higher. Studies on twins have shown a 62-70% consistency between monozygotic twins with respect to 21-23% of dizygotic twins. The mechanism of transmission of these diseases is apparently linked to many factors and the risk of a recurrence of PsA seems much higher compared to that of psoriasis. The risk of recurrence in a person with a first-degree relative suffering from psoriasis or Psoriatic Arthritis is 7.6 for the former and 30.4 for the latter. These data confirm the great importance of the genetic component behind the pathogenesis of these diseases. Many complex studies are required to understand exactly the genetic components behind these diseases.
Family studies and studies on twins have clearly shown that both psoriasis and psoriatic arthritis have a great genetic component. People with a fist degree relative suffering from psoriasis are much more likely to start suffering from the disease if compared to the general population. In particular, the risk of psoriasis in patients’ children is 4 to 10 times higher. Studies on twins have shown a 62-70% consistency between monozygotic twins with respect to 21-23% of dizygotic twins. The mechanism of transmission of these diseases is apparently linked to many factors and the risk of a recurrence of PsA seems much higher compared to that of psoriasis. The risk of recurrence in a person with a first-degree relative suffering from psoriasis or Psoriatic Arthritis is 7.6 for the former and 30.4 for the latter. These data confirm the great importance of the genetic component behind the pathogenesis of these diseases. Many complex studies are required to understand exactly the genetic components behind these diseases.
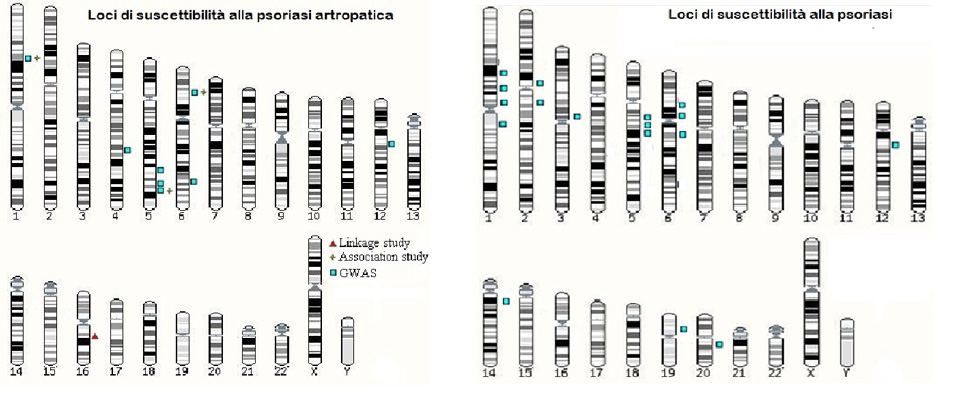
Many research groups have focused in these years on psoriasis and have highlighted many different psoriasis susceptibility loci (PSORS), i.e. DNA points capable of influencing directly or indirectly the development of the disease. Among the loci associated with psoriasis, there are: PSORS1 on the short arm of chromosome 6 (6p21.3), PSORS2 on the long arm of chromosome 17 (17q), PSORS4 on the long arm of chromosome 1 (1q21.3), PSORS5 on the long arm of chromosome 3 (3q21), PSORS6 on the short arm of chromosome 19 (19p), PSORS7 on the short arm of chromosome 1 (1p), PSORS8 on the long arm of chromosome 16 (16q), PSORS9 on the long arm of chromosome 4 (4q28-32) and PSORS10 on the short arm of chromosome 18 (18p11). The presence of all these loci is evidence of the great amount of studies made to discover them, but if we actually investigate their weight on influencing the risk to develop psoriasis, we will see it is very little. This makes us deduct that the role of genetics is linked to a great number of variables that, if taken singularly have little effect. This specific genetic scenario behind psoriasis does not allow us to establish a valid genetic test to define each individual risk.
For what concerns, psoriatic arthritis, we know a lot less about its genetic basis. Psoriasis and Psoriatic Arthritis are both associated with locus PSORS1, localised in chromosome 6 near the region responsible for codifying the greater system of histocompatibility. This region contains some of the most important genes responsible for immune response. The first evidence of the association between psoriasis and the HLA system were discovered in the 70s and then confirmed later on with the introduction of new techniques of analysis. The first genes to be identified were HLA-C, in particular HLA-Cw6 and HLA-B. Researchers then discovered that the association with HLA-B was merely due to a linkage disequilibrium with HLA-C. Linkage disequilibrium is the tendency that some genetic variants have to be inherited together. Significant associations were found also with PsA. In particular, whilst HLA-B*13, *16, *38, *39, *17 and HLA-Cw6 may have an effect on the onset of psoriasis (or of PsA), HLA-B*27 and *7 are specifically associated to PsA, and especially to those forms linked to an early onset of psoriasis.
 Great progress has been made in understanding the mechanisms behind psoriasis and psoriatic arthritis; many genetic and environmental factors have been identified and current medical treatments can take into account many different environmental factors. For what concerns genetic factors, we have seen that despite the many studies made, we still know very little. Currently identified loci can explain only a small fraction of the estimated inheritance, thus only a very little part of the risk due to the fact one has a first-degree relative suffering from the disease is linked to the presence/absence of oversensitivity/protection loci. In the near future, the progress of research and the introduction of new techniques capable of analysing not only genomic variants, but also epigenetic variants, will allow us to identify new factors and clarify the specific role of the factors we already know. Progress in the research on psoriasis and psoriatic arthritis will improve our knowledge on the origin of these diseases and help foresee the onset and development as well as one’s response to treatments. This could make us develop specific tailor-made treatments based on data that can be easily obtained, such as DNA and environmental factors to which the patients is exposed.
Great progress has been made in understanding the mechanisms behind psoriasis and psoriatic arthritis; many genetic and environmental factors have been identified and current medical treatments can take into account many different environmental factors. For what concerns genetic factors, we have seen that despite the many studies made, we still know very little. Currently identified loci can explain only a small fraction of the estimated inheritance, thus only a very little part of the risk due to the fact one has a first-degree relative suffering from the disease is linked to the presence/absence of oversensitivity/protection loci. In the near future, the progress of research and the introduction of new techniques capable of analysing not only genomic variants, but also epigenetic variants, will allow us to identify new factors and clarify the specific role of the factors we already know. Progress in the research on psoriasis and psoriatic arthritis will improve our knowledge on the origin of these diseases and help foresee the onset and development as well as one’s response to treatments. This could make us develop specific tailor-made treatments based on data that can be easily obtained, such as DNA and environmental factors to which the patients is exposed.
ADIPSO’S SUPPORT TO GENETIC RESEARCH
ADIPSO has always believed in genetic research and in the potentials of genetics as a tool to prevent and treat psoriasis. ADIPSO’s support to the research group headed by Prof. Giusepe Novelli of the University of Rome ‘Tor Vergata’ has allowed the group to carry out important genetic studies aimed at understanding the molecular basis of the disease. A very important discovery was the one that established that the region named 1q21 on chromosome 1 contains very important genes for the development of the disease. More specifically, we have seen that this region is fundamental in particular for Italian patients; this has established the basis for a personalised genetic medicine. Further studies financed by ADIPSO have identified the oversensitivity genes for psoriasis present on this chromosome. Great results have also been obtained to evaluate the genetic component of psoriatic arthritis. More specifically, all main genes involved have been identified through a great study that saw the participation/collaboration of the most important research groups on psoriasis at global level. Italy participated thanks to ADIPSO’s support.
At the end of the 70s, researchers made random studies on specific systemic drugs for the treatment of psoriasis that demonstrated that psoriasis is an immuno-mediated disease that involves the immune system. It defends us from all external bodies that enter into our immune system. For what concerns psoriasis, this occurs through the mechanism of the immune-mediated cell that basically involves two types of cells: lymphocytes T and APCs (Antigen Presenting Cells)
Lymphocytes T are "sentinel cells" that react when before an antigen by attacking it and destroying it. This action is mediated by APCs that are specific cells that capture the antigens and ‘lead’ them to lymphocytes so that the latter can destroy them.
The immune process foresees three steps:
- Lymphocytes T are not able at birth to recognise any kind of antigen, thus they need to be ‘trained’. APCs are responsible for their training. The external structure of the lymphocyte changes so that from that moment on they will recognise that specific antigen.
- Once ‘trained’, lymphocytes T migrate into the blood and move all around the body through our blood vessels.
- Once lymphocytes T have reached the peripheral areas of our body, they begin ‘hunting’ antigens. If they meet the antigens they have been trained to attack, they react by reproducing themselves, attacking the intruder and eliminating it.
In psoriasis when lymphocytes get into the blood, they come out of the vessels and go towards the skin where they meet again the antigen, activate themselves and react by producing cytokines.
When they get in contact with the epidermidis, cytokines increase the division speed up to seven times, whilst causing an inflammation in the derma.
The excessive growth of the lower parts of the skin does not allow the upper parts to fully mature.
The final result is that thicker layers of cells accumulate and thus form a plaque.
Research is currently oriented towards the production of protein molecules obtained through recombinant DNA techniques.
How they work
Before being marketed, all new drugs must undergo a long trial period. In general, we talk about clinical trials when one wants to test the efficacy and/or tolerability and/or safety of a drug on humans.
Clinical trials are long and expensive. The different phases are established by law so that we can guarantee ethical procedures that minimise the risk for patients.
The most used term for these experiments is clinical trials.
Where clinical trials on new drugs are performed
Clinical Trials are generally carried out in hospitals/authorised public or private universities.
The company or organisation financing the study is called the sponsor.
Sponsors of clinical trials are mostly pharmaceutical industries, interested in developing new drugs they wish to market. That is why they invest huge amounts of money, since clinical trials are long and expensive.
A minor part of clinical trials are sponsored by public research bodies.
A multicentre study involves several institutes or research centres.
Controlled clinical studies
In controlled clinical studies, a group of patients receives the experimental drug/treatment, while the other group – the so-called control group – receives a standard treatment – e.g. a drug already used for this kind of disease – or – if considered ethically/clinically acceptable – a placebo, i.e. a preparation apparently similar to the tested one, but with no active principle. In this way, the efficacy of the new drug is compared with that of a standard treatment or placebo.
In a randomised controlled study, patients are randomly assigned to the experimental or control group rather than being selected by those performing the study.
Blind and double blind studies
A randomised study is blind when patients do not know which group they belong to.
In a double blind study, neither patients nor doctors know who is receiving the experimental drug and who is receiving the placebo. The labels of both the drug and the placebo have codes that are then revealed at the end of the study, or in case of need.
In a double blind study, the efficacy of the pharmacological treatment is evaluated by comparing the data of the patients treated with the drug and those of the patients treated with the placebo. Only in case of a statistically significant difference in favour of the group of patients that has been treated with the drug, one can say that the latter is effective.
The different steps of clinical trials
Establishing a clear distinction between the different steps of clinical trials is not always simple, since phases may overlap depending on the kind of product or study method. Clinical trials have generally four phases. At the end of each phase, one can foresee whether the drug can enter into the next phase or not.
Phase I
Preliminary study on the safety and action method
The main purpose of this first phase is not to assess the efficacy of the new drug, but rather to establish its safety and, at the same time, understand what it causes in the human body, i.e. how it is absorbed, metabolised and excreted.
This kind of study is generally made on a small group of healthy voluntary patients. Phase I can also be useful to highlight possible undesired effects linked to dosage.
For a drug to access the next step, it is necessary it has proved to be not toxic or, at least, to have an acceptable level of toxicity with respect to its use.
Phase II
Pilot studies
The main purpose is to assess the efficacy of the drug at a given dosage and defined posology on a limited number of patients suffering from the disease or clinical condition for which the drug is being suggested.
Phase III
Large scale studies
If phase II gives encouraging results, phase III will foresee a greater number of patients in order to analyse in detail the efficacy data, establish the most suitable dosage and monitor possible side effects on a more statistically significant sample of patients.
In most cases, phase III studies are randomised and double blind; their duration varies according to the goals the study has foreseen. During this phase, great attention is given to tolerability- i.e. undesired and/or side effects.
Drugs that pass successfully phase III are given marketing authorisation.
Phase IV
Post-marketing
Even after a drug has been sold and used by thousands of people in one or more countries, clinical studies continue with the so-called phase IV.
Phase IV studies aim at confirming the long-term safety and tolerability of the drug on a greater sample of patients.
IThe data obtained are statistically relevant as they involve a great number of users, often different for age, race, gender, etc.
Authorities monitoring Clinical Trials
Clinical trials are monitored by public health authorities – e.g. the National Health Institute, the Ministry of Health, Regional Ethic Committees, Local Ethic Committees - and regulated by the law.
For what concerns EU countries, EMEA works to coordinate and harmonise all procedures within EU member countries.
The authority in charge in the US is the FDA (Food and Drug Administration)
Authorisations required for clinical trials
It is interesting to know that of all new potentially useful molecules, only 1 on 40,000 will actually become a drug.
 Family studies and studies on twins have clearly shown that both psoriasis and psoriatic arthritis have a great genetic component. People with a fist degree relative suffering from psoriasis are much more likely to start suffering from the disease if compared to the general population. In particular, the risk of psoriasis in patients’ children is 4 to 10 times higher. Studies on twins have shown a 62-70% consistency between monozygotic twins with respect to 21-23% of dizygotic twins. The mechanism of transmission of these diseases is apparently linked to many factors and the risk of a recurrence of PsA seems much higher compared to that of psoriasis. The risk of recurrence in a person with a first-degree relative suffering from psoriasis or Psoriatic Arthritis is 7.6 for the former and 30.4 for the latter. These data confirm the great importance of the genetic component behind the pathogenesis of these diseases. Many complex studies are required to understand exactly the genetic components behind these diseases.
Family studies and studies on twins have clearly shown that both psoriasis and psoriatic arthritis have a great genetic component. People with a fist degree relative suffering from psoriasis are much more likely to start suffering from the disease if compared to the general population. In particular, the risk of psoriasis in patients’ children is 4 to 10 times higher. Studies on twins have shown a 62-70% consistency between monozygotic twins with respect to 21-23% of dizygotic twins. The mechanism of transmission of these diseases is apparently linked to many factors and the risk of a recurrence of PsA seems much higher compared to that of psoriasis. The risk of recurrence in a person with a first-degree relative suffering from psoriasis or Psoriatic Arthritis is 7.6 for the former and 30.4 for the latter. These data confirm the great importance of the genetic component behind the pathogenesis of these diseases. Many complex studies are required to understand exactly the genetic components behind these diseases. Great progress has been made in understanding the mechanisms behind psoriasis and psoriatic arthritis; many genetic and environmental factors have been identified and current medical treatments can take into account many different environmental factors. For what concerns genetic factors, we have seen that despite the many studies made, we still know very little. Currently identified loci can explain only a small fraction of the estimated inheritance, thus only a very little part of the risk due to the fact one has a first-degree relative suffering from the disease is linked to the presence/absence of oversensitivity/protection loci. In the near future, the progress of research and the introduction of new techniques capable of analysing not only genomic variants, but also epigenetic variants, will allow us to identify new factors and clarify the specific role of the factors we already know. Progress in the research on psoriasis and psoriatic arthritis will improve our knowledge on the origin of these diseases and help foresee the onset and development as well as one’s response to treatments. This could make us develop specific tailor-made treatments based on data that can be easily obtained, such as DNA and environmental factors to which the patients is exposed.
Great progress has been made in understanding the mechanisms behind psoriasis and psoriatic arthritis; many genetic and environmental factors have been identified and current medical treatments can take into account many different environmental factors. For what concerns genetic factors, we have seen that despite the many studies made, we still know very little. Currently identified loci can explain only a small fraction of the estimated inheritance, thus only a very little part of the risk due to the fact one has a first-degree relative suffering from the disease is linked to the presence/absence of oversensitivity/protection loci. In the near future, the progress of research and the introduction of new techniques capable of analysing not only genomic variants, but also epigenetic variants, will allow us to identify new factors and clarify the specific role of the factors we already know. Progress in the research on psoriasis and psoriatic arthritis will improve our knowledge on the origin of these diseases and help foresee the onset and development as well as one’s response to treatments. This could make us develop specific tailor-made treatments based on data that can be easily obtained, such as DNA and environmental factors to which the patients is exposed.


